Amino acids
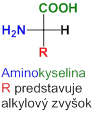
As we can see in the general formula of amino acids, their α-carbon is the chiral center of the molecule because it has four different substituents, which can be arranged differently in space even though the amino acid’s empirical formula remains the same. Of course, glycine is an exception; it has two identical substituents—hydrogens—on this carbon.
Amino acids in proteins have the L-configuration, which means that in this special notation, the amino acid is shown on the left:
D-amino acids are considered “non-natural” and have been found only in some peptide antibiotics and in the cell walls of certain bacteria.
To make amino acids easier to work with, scientists assigned each proteinogenic amino acid a three-letter and a one-letter code.
| AA | 3-letter code | 1-letter code |
| Nonpolar hydrophobic | ||
| glycine | Gly | G |
| alanine | Ala | A |
| valine | Val | V |
| leucine | Leu | L |
| isoleucine | Ile | I |
| methionine | Met | M |
| phenylalanine | Phe | F |
| tryptophan | Trp | W |
| proline | Pro | P |
| AA | 3-letter code | 1-letter code | |
| polar negatively charged | |||
| lysine | Lys | K | |
| arginine | Arg | R | |
| histidine | His | H | |
| polar positively charged | |||
| aspartic acid | Asp | D | |
| glutamic acid | Glu | E | |
| | | | |
| AA | 3-letter code | 1-letter code |
| polar hydrophilic | ||
| serine | Ser | S |
| threonine | Thr | T |
| cysteine | Cys | C |
| tyrosine | Tyr | Y |
| asparagine | Asn | N |
| glutamine | Gln | Q |
In solution, the amino group is intramolecularly (i.e., within the same molecule) neutralized by a proton from the carboxyl group. A so-called zwitterion forms (an ion that has both a positive and a negative charge at the same time, also called an amphion). If an amino acid is in an acidic solution rich in H+ ions (low pH), the carboxyl group becomes further protonated. The amino acid thus enters the protonated state, in which it has an overall positive charge. Conversely, if a zwitterion enters a solution with high pH, i.e., a basic solution, the NH3+ group is deprotonated (loss of H+), and the amino acid enters the deprotonated state, in which it is negatively charged.
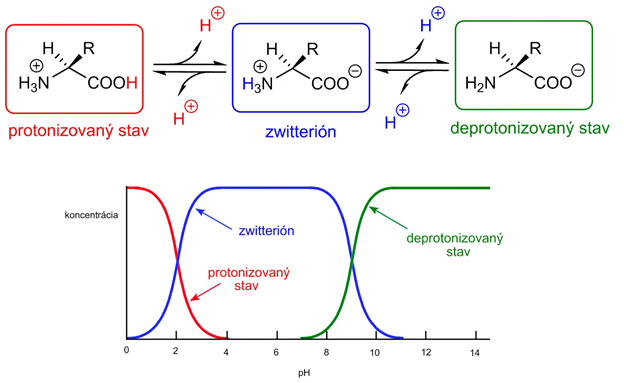
Figure – concentrations of ionization states of an amino acid as a function of solution pH.
In a basic solution with high pH, amino acids are present and behave as anions. In an acidic solution with low pH, they behave as cations. The pH value at which an amino acid (and also a peptide or protein) is neutral and occurs in the form of a zwitterion is called the isoelectric point and is different for each amino acid (peptide/protein).
Fun fact:
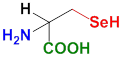
There is one more proteinogenic amino acid, selenocysteine, which has a selenium atom in place of the sulfur in the –SH group. Unlike cysteine, it has a lower pKa and a higher reduction potential, and therefore is part of antioxidant selenoproteins such as glutathione peroxidase, thioredoxin reductase, formate dehydrogenase, glycine reductase, and some hydrogenases. For it to be incorporated into the peptide chain of these enzymes, it must be specially encoded in DNA and preceded by an approximately 60-nucleotide-long sequence called the SECIS element (from English: SelenoCysteine Insertion Sequence element).
1. Assign the correct type of alkyl side chain to the amino acid:
a) lysine 1) basic
b) serine 2) acidic
c) cysteine 3) contains oxygen
d) leucine 4) nonpolar aliphatic
e) aspartic acid 5) contains sulfur
Solution:
- a-1
- b-2
- c-5
- d-4
- e-2
2.
At what pH are these amino acids found?
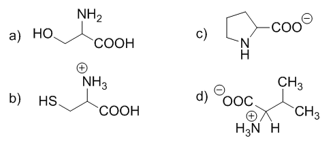
Solution:
a) The molecule as a whole is neutral; neither group is protonated or deprotonated. It is at neutral pH.
b) The molecule contains an undissociated carboxyl group and a protonated amino group. Therefore, it is in an environment rich in H+ ions, i.e., in an acidic environment with low pH.
c) The amino acid has a deprotonated carboxyl group; therefore, it is in a basic environment with high pH.
d) The molecule is present in solution as a zwitterion; therefore, it is at its isoelectric point, which for the valine shown is pH 6.02.
3. Characterize the amino acid shown:
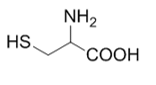
The amino acid contains one amino group and one carboxyl group, both in their neutral forms. The thiol group in the side chain does not affect the acidic or basic properties of the molecule.
4. Which of the following amino acids is not optically active?
- a) threonine
- b) valine
- c) serine
- d) glutamine
- e) glycine
- f) lysine
5.How many amino acids commonly occur in proteins?
- a) 300
- b) 20
- c) 40
- d) 5
6. All of the following amino acids are aliphatic except:
- a) glycine
- b) histidine
- c) lysine
- d) alanine
- e) aspartic acid
- f) proline
7.Which statement about the isoelectric point is correct:
- a) the pH at which an amino acid does not move in an electric field
- b) the pH at which an amino acid moves toward the cathode
- c) the pH at which an amino acid moves toward the anode
- d) the pH at which an amino acid occurs in the form of an amphion (zwitterion)
8.Which statement about the isoelectric point is correct:
- a) the isoelectric point expresses a specific pH value
- b) the isoelectric point depends on the functional groups of the molecule
- c) the isoelectric point depends on the molecular weight of the amino acid
- d) only amino acids have an isoelectric point
- e) the isoelectric point is the same for all proteins
- f) the isoelectric point is a characteristic feature of each amino acid
9. Aspartic acid is:
- a) a monoamino dicarboxylic acid
- b) a diamino monocarboxylic acid
- c) an aromatic amino acid
- d) an imino acid
10. List and draw the sulfur-containing amino acids.
Please log in to view the solution.