Density
1.Calculate the density of nitrogen in g.dm-3 under normal conditions.
Solution:
We substitute the values from the task and from tables into the equation for calculating density from the state equation.
Ar (N)=14.01
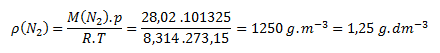
The density of nitrogen N2 under normal conditions is 1.25 g.dm-3.
2.Calculate the density of mercury in g.cm-3 at 20°C, if the molar volume of mercury at this temperature is 0.0148 dm3.mol-1.
Solution:
From tables we find M(Hg) = 200.59 g.mol-1. Substitute the values into the formula:
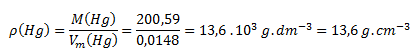
The density of mercury at 20°C is 13.6 g.cm-3.
3.Determine the molar mass of a gas that has a density of 1.429 kg.m-3 under normal conditions.
Solution:
From the density we can logically infer that 1000 liters of gas has a mass of 1.429 kg. Under normal conditions 1 mol of gas occupies 22.41 liters.
1000 l of gas.......................1.429 kg
22.41 l of gas......................x kg
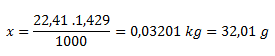
The molar mass of the unknown gas is 32.01 g.mol-1. From tables we see that it is oxygen.
4.It was measured that the density of an unknown gaseous saturated hydrocarbon is 21.88 times higher than the density of hydrogen under the same experimental conditions (pressure and temperature). Calculate the molar mass of the hydrocarbon and identify it using tables.
Please log in to view the solution.
5.Calculate the density in kg.m-3
- a) oxygen O2 under normal conditions
- b) ozone O3 under normal conditions
6.1 m3 of nitrogen has a mass of 1.25 kg under normal conditions. At what temperature and constant pressure will the density of nitrogen be halved?
Please log in to view the solution.
7.Calculate the relative molecular mass of a gaseous substance whose relative density compared to air is 1.586.
Please log in to view the solution.8.What is the relative density of natural gas compared to air, if this gas contains 75 vol.% CH4, 15 vol.% C2H4, 7 vol.% H2, 1 vol.% CO and 2 vol.% CO2.
Please log in to view the solution.9.1 m3 of oxygen has a mass of 1.43 kg at 0°C and a pressure of 101.325 kPa. Calculate the density of oxygen at 23°C and a pressure of 95.99 kPa.
Please log in to view the solution.10.1 m3 of gas has a mass of 1.33 kg at 100 kPa and 17°C. What will be the mass of 500 ml of the gas at 85.33 kPa and 37°C?
Please log in to view the solution.