Atomic nucleus
1. What properties does the atomic nucleus have?
Solution:
The atomic nucleus forms the central part of the atom. It accounts for almost all of its mass. The atomic nucleus contains protons and neutrons—nucleons.
Atomic constant:
Proton:
Neutron:
proton number (number of protons in the nucleus, electrons in the shell, the order of the element in the Mendeleev table)
neutron number (number of neutrons in the nucleus)
nucleon number (number of nucleons – protons and neutrons in the nucleus)
Isotopes are nuclides of the same element (same ) that differ in neutron number (and thus also in number ).
The mass of the nucleus is always smaller than the mass of protons and neutrons.
mass defect of the nucleus:
Binding energy of the nucleus:
Binding energy per nucleon:
New unit of mass:
2.Lead has four isotopes. Determine the number of protons and neutrons in the nucleus.
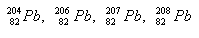
Solution:
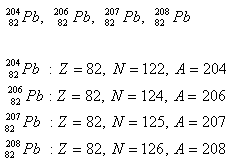
3. Calculate:
- a.) the rest mass of the nitrogen nucleus, given Ar(N) = 14,0067
- b.) the relative mass of the chlorine nucleus, given m(Cl) = 5,885·10–26 kg, 1 MeV·c–2 = 1,7825·10–30 kg, mu = 1,6605·10–27 kg
Solution:
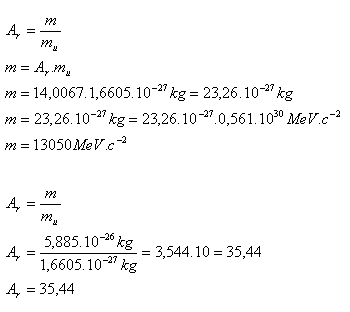
- The rest mass of the nitrogen nucleus is m = 13 050 MeV·c-2.
- The relative mass of the chlorine nucleus is Ar = 35.44.
4.Determine the mass defect, the binding energy, and the energy per nucleon for a radium nucleus. Express the energy in MeV.
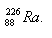
5.Determine the percent composition of chlorine with a relative atomic mass of 35.5, which is a mixture of isotopes
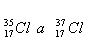
6.In the atomic bomb dropped by the Americans on Hiroshima, the charge contained 44.5 kg of uranium. What energy was released?
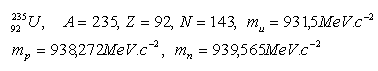
7.How long would it take a power plant, whose two blocks have a power output of P = 2,440 MW, to produce the energy from problem no. 6 (E = 3.24·1016 J)?
Please log in to view the solution.
8.A nucleus with mass number 100 and binding energy εj1 = 7.4 MeV splits into two nuclei with binding energy per nucleon εj2 = 8.2 MeV. What energy is released in the reaction?
Please log in to view the solution.
9. Determine the energy obtainable by fission of 1 kg of uranium 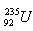 if fission of one uranium nucleus releases 200 MeV of energy. What mass of hard coal with a heating value of 3·107 J·kg would be needed to obtain the same energy?
if fission of one uranium nucleus releases 200 MeV of energy. What mass of hard coal with a heating value of 3·107 J·kg would be needed to obtain the same energy?
10.A block of a nuclear power plant that converts nuclear energy into electrical energy with 40% efficiency has an electrical output of 600 MW. Determine the mass of uranium 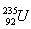 consumed in 24 hours if fission of a single nucleus releases energy of 200 MeV.
consumed in 24 hours if fission of a single nucleus releases energy of 200 MeV.
Please log in to view the solution.
11.Show that for the units of mass used in atomic physics, the following holds:
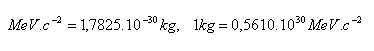
12.Express, in units of MeV c–2, the mass of the atomic mass constant mu, the proton mp, and the neutron mn.
Please log in to view the solution.13.Determine the binding energy of the carbon nucleus per nucleon.
Please log in to view the solution.14.Determine the number of uranium atoms in 1 kg of uranium.
Please log in to view the solution.15.Find how many protons and how many neutrons are in 1 kg of neon.
Please log in to view the solution.16.Determine the total binding energy of nuclei in one kilogram of uranium. One kilogram of uranium contains 2.53×1024 atoms.
Please log in to view the solution.17.Calculate the magnitude of the electric force
- a.) by which two protons in a nucleus repel each other (r = 10–15 m)
- b.) by which a proton and an electron attract each other (r = 10–10 m)
18.A nucleus with mass number A = 230 and binding energy per nucleon
εj1 = 7.4 MeV splits into two nuclei with binding energy per nucleon εj2 = 8.3 MeV. What energy is released during fission?
19.Boron, as it occurs in nature, is a mixture of two isotopes. The average atomic mass of boron is 10.82 mu. What percentage of each isotope is in natural boron if:
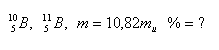
20.Compare the density of the hydrogen nucleus with the density of water.
Please log in to view the solution.