State equation
1. What is the relationship between pressure, volume and temperature of an ideal gas?
Solution:
The state of an ideal gas in thermodynamic equilibrium is described by three state variables: pressure p, volume V and thermodynamic temperature T. The mutual relationship between these state variables is expressed by the equation of state for an ideal gas.
The equation of state for an ideal gas:
a) For two states (at constant mass of gas):
b) For one mole:
c) For n moles:
d) For N molecules:
e) For m kilograms:
f) For a mixture of chemically non-reacting gases:
2.Derive the numerical value and the dimension of the molar gas constant Rm.
Solution:
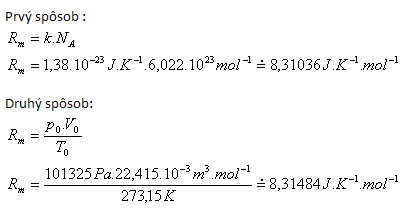
Tabulated value Rm = 3.31441 J·K-1·mol-1.
Differences are caused by different rounding of input values.
3.Find how many molecules are contained in 1 cm3 of any gas under normal conditions.
Solution:
Analysis:
V = 1 cm3 = 10-6 m3, p = 101325 Pa, T = 273.15 K
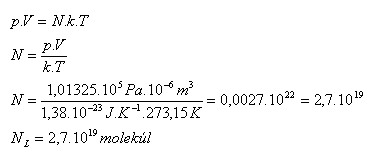
One cubic centimeter of any gas under standard conditions contains NL = 2.7·1019 molecules. This is Loschmidt's number. (Johann Josef Loschmidt 1821–1895)
4. In a vessel with internal volume 8.3 m3 there is hydrogen H2 with mass 200 g and temperature 27oC. Determine its pressure!
Please log in to view the solution.
5.In a vessel with internal volume 5·10-3 m3 there is nitrogen N2 at temperature 39oC and pressure 1.6·105 Pa. Determine its mass.
Please log in to view the solution.
6.What is the pressure of air in a truck tire at temperature 20oC and density 8 kg·m-3. Mm(air) = 29·10-3 kg·mol-1
Please log in to view the solution.7.How many molecules are in a spherical vessel of internal radius 3 cm, filled with oxygen O2 at temperature 27oC and pressure 1.36·10-2 Pa.
Please log in to view the solution.8.In a vessel there is nitrogen N2 with mass 10 kg at pressure 10 MPa. Determine the mass of nitrogen that must be released from the vessel so that its pressure decreases to 2.5 MPa. (The temperature of the nitrogen does not change)
Please log in to view the solution.
9.The density of nitrogen under standard conditions (T1 = 273.15 K and p1 = 101325 Pa) is ρ1 = 1.23 kg·m-3. Determine the density of nitrogen at temperature 30oC and normal pressure.
Please log in to view the solution.
10. Calculate the effective molar mass of air Mm. Consider air as a mixture of 75% nitrogen and 25% oxygen.
Please log in to view the solution.
11.An ideal gas enclosed in a container with an internal volume of 2.5 liters has a temperature of -13.150C. What is its pressure if the gas contains 1024 molecules?
Please log in to view the solution.
12.Determine the volume of carbon dioxide CO2 with a mass of 1 gram at a temperature of 210C and a pressure of 1.0 kPa.
Please log in to view the solution.
13.How many molecules are contained in a container with a gas that has an internal volume of 1 liter, if the gas has a temperature of 100C and a pressure of 0.2 MPa?
Please log in to view the solution.
14.An ideal gas enclosed in a container has a volume of 1.3 m3 and a temperature of -13.150C. What is the pressure of this gas if its amount of substance is 4 kilomoles?
Please log in to view the solution.
15.Determine the volume of oxygen O2 with a mass of 8 grams at a temperature of 210C and a pressure of 1.4·105 Pa.
Please log in to view the solution.
16.In a container with a volume of 5·10-3 m3, nitrogen N2 is enclosed at a temperature of 390C and a pressure of 1.6·105 Pa. Determine the mass of the nitrogen.
Please log in to view the solution.
17.Determine the molar mass of a gas which, at a temperature of 00C and a pressure of 100 kPa, has a density of 1.95 kg·m-3. Which gas is it?
Please log in to view the solution.
18.The density of air under normal conditions is 1.27 kg·m-3. Determine the density of air at a temperature of 300C and normal pressure.
Please log in to view the solution.
19.How does the volume of an ideal gas change when its thermodynamic temperature is doubled and the pressure increases by 25%?
Please log in to view the solution.
20.Show that any ideal gas has, under normal conditions ( p0 = 101325 Pa, T0 = 273.15 K ), the same molar volume V0 = 22.4 liters·mol-1.
Please log in to view the solution.