Amount of substance
1. Calculate the number of nitric oxide molecules in a container that contains 4.5 mol of this gas.
Solution:
We start from the equation for calculating the amount of substance:
N(NO) = n(NO).NA = 4.5 mol × 6.022 × 1023 = 2.7 × 1024
The container contains 2.7 × 1024 NO molecules.
2. What amount of substance corresponds to 1.806 × 1027 carbon atoms?
Solution:
We use the relation for the amount of substance.
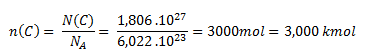
The amount of carbon substance containing 1.806 × 1027 atoms is 3 kmol.
3. How many molecules are in a container containing 2.5 mol of carbon dioxide?
Solution:
We use a similar procedure as in example 2.
1.5 × 1024 molecules.
4. Calculate the amount of substance:
- a) 2.58 × 1027 atoms of aluminum
- b) 5.55 × 1019 sodium cations
- c) 8.68 × 1022 chloride anions
- d) 1.75 × 1025 molecules of white phosphorus (P4)
5. The charge of an electron is 1.6 × 10-19 C. What is the charge of 1 mol of electrons (Faraday’s constant)?
Please log in to view the solution.
6. How many silver atoms are contained in 1 cm3 of this metal, which has a density of ρ = 10.5 × 103 kg·m-3?
Please log in to view the solution.
7. What amount of substance of H2O is in one liter of water?
Please log in to view the solution.8. What amount of sodium is in 10 g of NaCl? How many chlorine atoms correspond to this amount of sodium in the compound?
Please log in to view the solution.9. What amount of substance corresponds to 1 g of H2SO4 and 200 g of CH4?
Please log in to view the solution.
10. What amount of aluminum is in a cube with an edge of 10 cm? (use data from chemical tables)
Please log in to view the solution.