Compositions of solutions
1. How many grams of calcium nitrate and water are in 130 g of a 20% solution?
Solution:
100 grams of solution ........... 20 g of nitrate
130 g ......................................... x g of nitrate
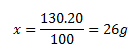
The mass of water is calculated as 130 − 26 = 104 g of water. The solution consists of 26 grams of calcium nitrate and 104 grams of water.
2. In 100 ml of water, 30 g of blue vitriol (copper(II) sulfate pentahydrate) were dissolved. What is the mass concentration of the solution thus prepared?
Solution:
First, it is necessary to calculate the amount of pure sulfate in blue vitriol.
M(CuSO4·5H2O) = 249.68 g·mol-1
M(CuSO4) = 159.60 g·mol-1
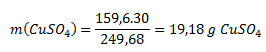
The mass of the solution is 100 g (water) + 30 g (blue vitriol) = 130 g. The concentration of the solution is therefore:
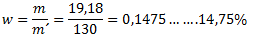
3. How many grams of sodium hydroxide are needed to prepare 5 liters of a 10% solution with density ρ = 1115 kg·m-3?
Solution:
We will use a combination of the formula for mass concentration and density:
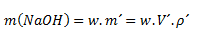
where m´ is the mass of the solution, V´ is the volume of the solution, and ρ´ is the density of the solution.
After substituting numerical values:
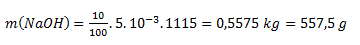
To prepare 5 liters of a 10% solution, we need 557.5 g of NaOH.
4. Calculate the molar concentration of a 22% sulfuric acid solution with density 1.1548 g·cm-3.
Please log in to view the solution.
5. An aqueous ethanol solution contains 90 ml of pure alcohol in 150 ml of solution. What is the volume percent and mass percent of the solution?
Please log in to view the solution.6. How many grams of potassium chloride and how many milliliters of water are needed to prepare 245 g of a 2.5% solution?
Please log in to view the solution.7. What is the mass of potassium permanganate present in 25 ml of a 0.02 molar solution?
M(KMnO4) = 158.342 g·mol-1.
8.
What amount concentration will a solution of ammonium iron(III) sulfate have if 1 ml of such a solution corresponds to 1 mg of Fe2O3? M(Fe2O3) = 159.7 g·mol-1.
Please log in to view the solution.
9. What is the mass fraction and percent composition of a solution that contains, in 10 ml of water, 1.02 of dissolved substance?
Please log in to view the solution.10. What are the amount concentration and mass concentration of a nitric acid solution with a mass percent of 40? ρ40 = 1.252 g·cm-3, Mr(HNO3) = 63.01.
Please log in to view the solution.```