calculation of fraction and percent of element in compound
1.A container holds a gas mixture containing 0,825 mol of sulfur dioxide, 4,29 mol of nitrogen, 0,055 mol of argon, and 0,33 mol of oxygen. Calculate the mole fractions of the individual components in the system.
Solution:
First, calculate the total amount of substance in the gas mixture:
n = (0,825 + 4,29 + 0,055 + 0,33) mol = 5,50 mol
The mole fractions of the components are calculated as follows:
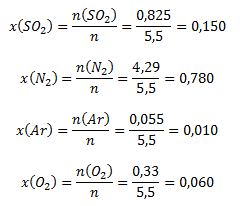
The mole fractions of the gases in the mixture are 0,15 (SO2); 0,78 (N2); 0,01 (Ar); 0,06 (O2).
2. Calculate:
- a) the mass fraction and percentage of sulfur and iron in pyrite
- b) how many grams of sulfur are contained in 500 g of pyrite
Solution:
a) The mass fraction of sulfur in pyrite is calculated according to:
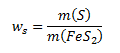
We rearrange this expression so that it contains the molar masses (taken from tables) and the amounts of substance of sulfur and pyrite. We therefore express the masses of the given substances as:
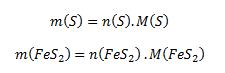
After substituting these relations into the equation for the mass fraction, we get:
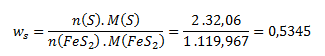
The calculated mass fraction of sulfur corresponds to the mass percent given by:
mass percent = wS·100 = 0,5345 · 100 = 53,45 %
The mass fraction of the second component of pyrite, iron, is then:
wFe = 1 – 0,5345 = 0,4655
mass percent = 0,4655 · 100 = 46,55 %
3.How many percent nitrogen does calcium nitrate Ca(NO3)2 contain?
Solution:
The molar mass of the nitrate is 164,086 g·mol-1. The relative atomic mass of nitrogen is 14,007. Logically, 164,086 grams of the compound contains 14,007 g of nitrogen. To find the percentage content, we take the ratio of the mass of nitrogen to the compound:
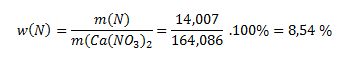
However, nitrogen occurs in nature as a dimer, N2, therefore the percentage content of N2 is double that of N; the correct answer is 17,07 %.
4.25 cm3 of glycerol was dissolved in 100 cm3 of water. Calculate the mass fraction of glycerol in the solution and express it in mass percent. The density of glycerol at 20°C is 1,261 g·cm-3.
Please log in to view the solution.5.At 25°C and a pressure of 101,5 kPa, 15,5 dm3 of hydrogen H2 and 10 dm3 of neon Ne were mixed. Express the composition of the gas mixture:
- a) in volume percent
- b) in mole percent
6.An alcoholic beverage contains 40 vol% ethanol. Calculate the volumes of pure ethanol and water in 750 cm3 of this beverage.
Please log in to view the solution.
7.How many grams of anhydrous copper(II) sulfate are contained in 12 g of its pentahydrate with a purity of 98,2%?
Please log in to view the solution.
8.How many percent sulfur does sulfur trioxide contain?
Please log in to view the solution.9.What percentage of its mass does green vitriol FeSO4·7H2O lose when heated to a high temperature (so that it loses all its crystallization water)?
Please log in to view the solution.10.Calculate the mass of a saturated solution of potassium dichromate at 50°C that was prepared from 10 g of pure dichromate. At 50°C, 34,5 g of K2Cr2O7 dissolve in 100 g of water.
Please log in to view the solution.