isoprocesses
1. What are isoprocesses?
Solution:
Isoprocesses are processes in an ideal gas with constant mass, during which one of the state variables remains constant.
A) Isothermal process (T = constant)
Boyle–Mariotte’s law
The heat received by an ideal gas during an isothermal process is used to perform work.
B) Isochoric process (V = constant)
Charles’s law
The heat received by an ideal gas in an isochoric process is used to increase its internal energy.
C) Isobaric process (p = constant)
Gay–Lussac’s law
The heat received by an ideal gas during an isobaric process is used both to change internal energy and to perform work.
D) Adiabatic process (Q = 0) – no heat exchange occurs between the gas and the surroundings. may change.
Poisson’s law
-
– specific heat capacity at constant pressure
-
– specific heat capacity at constant volume
2.What volume will 30 liters of air have if, at the same temperature, we increase its pressure from 72 kPa to 75 kPa?
Solution:
Analysis:
V1 = 30 l = 30·10-3 m3, p1 = 72·103 Pa, p2 = 75·103 Pa, V2 = ?
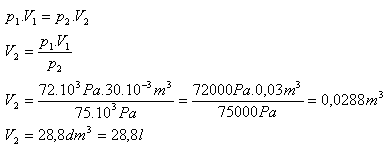
After increasing the pressure the air will have a volume of 28.8 liters.
3.The density of helium at pressure 105 Pa is ρ1 = 0.164 kg·m-3. What will its density be if we compress it to a pressure of 5·107 Pa? (The mass and temperature of the gas do not change during compression).
Solution:
Analysis:
p1 = 105 Pa, ρ1 = 0.164 kg·m-3 , p2 = 5·107 Pa, ρ2 = ?
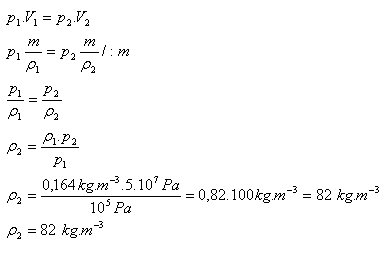
The density of helium after compression will be 82 kg·m-3.
4.The density of air under normal conditions is ρ1 = 1.3 kg·m-3. What will the density of the air be if we heat it from normal conditions to 30oC? (The amount of air does not change).
Please log in to view the solution.
5.A gas enclosed in a container with a movable piston is heated at constant pressure from 22oC to 52oC. By what percentage did its volume increase?
Please log in to view the solution.
6. A bulb is filled with nitrogen during manufacturing at a pressure of 50.6 kPa and a temperature of 18oC. What is the temperature of the nitrogen in the lit bulb if its pressure increases to 118 kPa?
Please log in to view the solution.
7.At a Formula 1 race, the temperature of the air in the tires increased from 19oC to 79oC. How does the pressure in the tire change if it was originally inflated to 240 kPa? Why is a "formation lap" performed before the start? (The internal volume of the tire does not change)
Please log in to view the solution.
8.In a cylindrical chamber there is air closed by a movable piston located 50 cm from the bottom of the cylinder. The air pressure is 105 Pa. If the piston is moved 20 cm toward the bottom during adiabatic compression, the air pressure increases to 2.05·105 Pa. Determine the Poisson constant for air!
Please log in to view the solution.
9.Show that for an adiabatic process T1·V1κ-1 = T2·V2κ-1
Please log in to view the solution.
10. For adiabatic compression the volume of the air was reduced to 1/10 of the original volume. Calculate the pressure and temperature of the air after adiabatic compression. The initial air pressure was 105 Pa, the initial temperature 20oC. κ (air) = 1.4
Please log in to view the solution.11.The pressure of a gas in a closed container at a temperature of 110C is 189 kPa. What will be the temperature of this gas if its pressure increases to 1 MPa?
Please log in to view the solution.
12.The pressure of a gas at a temperature of 200C is 107 kPa. What will its pressure be if its temperature increases to 1500C?
Please log in to view the solution.
13.The temperature of oxygen with a given mass increases isobarically from an initial temperature of -200C. At what temperature does the oxygen have 1.5 times the volume it had at the initial temperature?
Please log in to view the solution.
14.What process is written in the table:

15.The volume of a gas is 25 liters with a pressure of 1 kPa. What will its pressure be if we reduce its volume to 20% of the original volume?
Please log in to view the solution.
16.A cylindrical container 30 cm long is closed with a movable piston. A gas in the container is under a pressure of 0.5 MPa. Determine its pressure if the internal volume of the container increases by moving the piston by 10 cm.
Please log in to view the solution.
17.The mean free path of a molecule 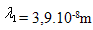 is at pressure p1 = 105Pa. What will the mean free path of the molecule be at a pressure of
is at pressure p1 = 105Pa. What will the mean free path of the molecule be at a pressure of
- a.) 1.0 Pa,
- b.) 107Pa.
18.Hydrogen H2 has a temperature of -30C. By adiabatic expansion its volume increases to three times the original volume. What is the resulting temperature of the hydrogen during this adiabatic process?
Please log in to view the solution.
19.Argon has, at normal pressure p1 = 101325 Pa, a volume V1 = 10 liters. After adiabatic compression its volume changes to V2 = 4 liters and the pressure to p2 = 0.468 MPa. Determine the Poisson constant for argon.
Please log in to view the solution.
20.Determine the specific heat capacity of argon at constant volume cV, if the specific heat capacity of argon at constant pressure is