Quantum optics
1. What is a photon?
Solution:
heories on the nature of light:
a.) Light is a mechanical wave of the ether. (Huygens, Young)
b.) Light is a stream of particles (corpuscles) emitted from the source. (Newton)
c.) Light is an electromagnetic wave with a frequency of about 3.8·10¹⁴ Hz to 7.8·10¹⁴ Hz, which propagates continuously from the source. (Maxwell)
d.) The energy of radiation (light) is not distributed continuously in space, but consists of a certain number of discrete packets of quanta, which can only be emitted and absorbed as whole units. A quantum of radiation (light) is called a photon. (Planck, Einstein)
Each photon has:
Energy:
Momentum
Wave–particle duality:
de Broglie: Every moving particle with mass and velocity has also a wave with wavelength
Compton: Each photon can be considered a massive particle with zero rest mass, which exists only at speed .
Compton experiment:
Photoelectric effect (photoemission) is the release of electrons from metals by incident radiation (photons):
-
internal (electrons are emitted from atoms but remain within the metal)
-
external (electrons are emitted from the surface of the metal and move in space with velocity )
Einstein’s equation of photoemission:
Franck–Hertz experiment proves that atoms absorb energy in quanta.
2. Calculate the energy of a photon corresponding to the extreme values of visible light. Violet has a wavelength λV=390 nm, red λR=790 nm. Express in joules and in eV. 1eV=1.602·10-19J.
Solution:
Analysis:
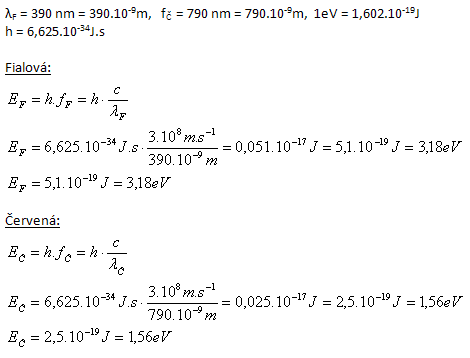
A violet light photon has energy 3.18 eV, red 1.56 eV.
3. Compare the energy of a photon of yellow monochromatic light (λ = 500·10-9m) with the average kinetic energy of a molecule of an ideal gas in its random motion at a temperature of 00C.
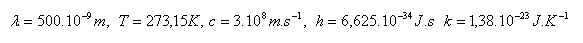
Solution:
Analysis:
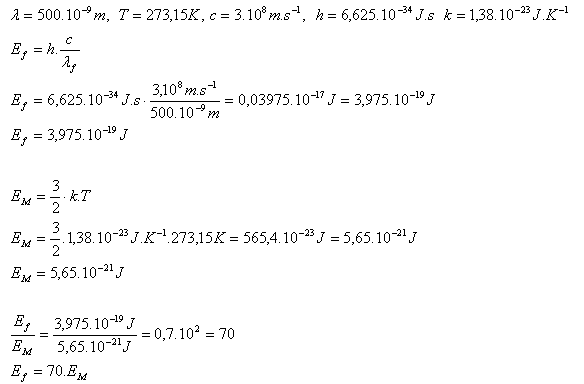
The energy of a yellow light photon is 70 times greater than the energy of a molecule of an ideal gas at 00C.
4.The human eye can perceive light if the power of the light radiation incident on the eye is at least P=2·10-17W. Determine how many photons with wavelength λ = 500·10-9m hit the eye in 1 second.
Please log in to view the solution.5.What is the threshold frequency of electromagnetic radiation needed to irradiate the surface of nickel so that the external photoelectric effect occurs? The work function of electrons from nickel is 5 eV.
Please log in to view the solution.6.Determine whether photoemission can occur when light with a wavelength λ = 390·10-9m falls on zinc.
7.At what speed do electrons leave a platinum plate (f0 = 12.8·1014Hz), if ultraviolet light with wavelength λ = 150nm falls on it? (me = 9.1·10-31kg)
Please log in to view the solution.8.The de Broglie wavelength of an accelerated electron is λ = 3.87·10-11m. The electron was accelerated from rest in an electric field with voltage U. Calculate:
- a) the speed of the electron
- b) the accelerating voltage
9.A photon of ultraviolet radiation has a wavelength λ = 100nm. Calculate
- a) how many photons have an energy of 1J
- b) how many photons have a mass of 1 microgram
10.In the Compton experiment, the incident photon has frequency f1=1.5·1020Hz, the photon after collision has frequency f2=1.1·1020Hz.
- a) Determine the energy gained by the electron that interacted with the photon. Express in eV
- b) Determine the change in wavelength of the photon.
11.In the Franck–Hertz experiment, the authors found that the current drop occurs at a voltage in the electric field accelerating the electrons equal to U = 4.9V and that mercury vapors emit radiation with wavelength λ = 253.2nm. Calculate the value of Planck’s constant h.
Please log in to view the solution.12. In the Compton effect of photon scattering on an electron, the photon wavelength changes by Δλ=4.85·10–12m. By what angle υ does the photon deviate from the original direction?
Please log in to view the solution.13. An X-ray tube operates with a voltage of 20 kV, current 10 mA and efficiency η = 0.2%. Calculate:
- a.) the short-wavelength limit of the continuous spectrum
- b.) the power radiated in the form of X-ray radiation
14.In a television receiver screen, electrons are accelerated by a potential difference of 15 kV. What is the speed and the de Broglie wavelength of these electrons?
Please log in to view the solution.15.In an electron microscope, electrons are emitted by a voltage of 105 V. What is the resolving power λ of this microscope?
Please log in to view the solution.16.What is the energy of a neutron whose wavelength is on the order of the distance between atoms in a crystal lattice
(10–10 m)?
17.How many photons of red light ( λ = 750 nm ) are needed to create a small sphere with a mass of
3 micrograms?
18.A light bulb emits visible light of frequency 5·1014 Hz. What is the energy and mass of one photon?
Please log in to view the solution.19.Compare the de Broglie wavelengths of an electron and a proton moving at the same speed.
Please log in to view the solution.20.A helium–neon laser is a source of monochromatic radiation with wavelength 632.8 nm. Its power is 2 mW. Determine the energy and mass of the photons of the laser radiation. How many photons are emitted per 1 second?
Please log in to view the solution.