Work of gas
1. Explain the work of a gas and the cyclic process.
Solution:
Hot gas or steam can perform work. Work of a gas at constant pressure:
A process in which the final state of the system is identical to the initial state is called a cyclic process.
Work done by a gas during one cycle:
-
= total heat absorbed by the gas during the cycle from the surroundings
-
= heat absorbed from the heater
-
= heat released to the cooler
Efficiency of a cyclic process and a heat engine (internal combustion engines, turbines, etc.):
Fuel calorific value (heating value):
2.Calculate the work of steam during one piston stroke if the steam pressure on the piston is 176.104 Pa and the piston area is 700 cm2. The piston stroke is 55 cm.
Solution:
Analysis:
p = 176.104 Pa, S = 700.10-4 m2, h = 55.10-2 m,
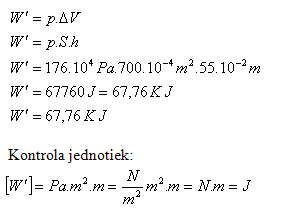
The work of steam during one piston stroke is W = 67.76 kJ
3.Oxygen O2 (Mm = 32.10-3 kg·mol-1) has a mass of 4 kg and a temperature of 0oC. How much will its temperature increase in an isobaric process if the gas performs work of 10.4 kJ?
Solution:
Analysis:
m = 4 kg, Mm = 32.10-3 kg·mol-1, W = 10.4·103 J, Rm = 8.314 J·kg-1·K-1
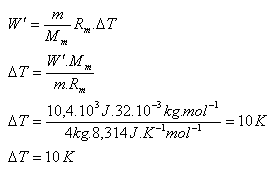
The gas temperature increases by ΔT = 10 K.
4. What is the mass of fuel consumed by airplane engines on a flight of 500 km if the average speed is 250 km·h-1, the average engine power is 2 MW, engine efficiency is 25%, and the fuel calorific value is 45 MJ·kg-1?
Please log in to view the solution.
5.A combustion engine receives heat of 1·104 J from a heater at 900oC. It transfers 75% of this heat to a cooler at 150oC. What is the engine efficiency? What is the maximum efficiency of an engine operating between these temperatures?
Please log in to view the solution.
6.What is the “second law of thermodynamics”?
Please log in to view the solution.
7. Complete the short test:
- 1.) The molar mass of carbon dioxide CO2 is: (12C, 16O)
A.) 44·10-3 kg·mol-1 B.) 55·10-3 kg·mol-1 C.) 33·10-3 kg·mol-1
- 2.) Which quantity does not belong among the basic state variables?
A.) temperature B.) volume C.) time
- 3.) An ideal gas has volume V at 27oC. At what temperature does it have volume 0.75V?
A.) –31oC B.) 48oC C.) –48oC
- 4.) An ideal gas has a pressure of 5 MPa at 0oC. Determine its pressure at –50oC.
A.) 3.04 MPa B.) 4.08 MPa C.) 5.03 MPa
- 5.) What is the calorific value of fuel if burning 200 kg of fuel releases 620·107 J of heat?
A.) 3.1·107 J·kg-1 B.) 5.4·106 J·kg-1 C.) 6.9·105 J·kg-1
- 6.) What is the maximum efficiency of a steam turbine if the heater temperature is 530oC and the cooler temperature is 50oC?
A.) 60 % B.) 65 % C.) 70 %
- 7.) What work does an ideal gas of 1 mol perform if its temperature increases by 1 K at constant pressure?
A.) 1.38 J B.) 8.314 J C.) 6.022 J
8.What work is done by the air if, at a constant pressure of 0.15 MPa, its volume increases from 3.2 liters to 5.2 liters?
Please log in to view the solution.
9.What work is done by air with a mass of 1.3 grams (Mm = 29·10-3 kg·mol-1) if, in an isobaric process, its temperature increases from 200C to 1000C?
Please log in to view the solution.
10.Determine the amount of substance of an ideal gas which, at constant pressure, when its temperature is increased by 20C, does 8314 J of work.
Please log in to view the solution.
11.In a heat engine, the gas received 0.5 MJ of heat from the heater and transferred 350 kJ of heat to the cooler.
- a.) What is the efficiency of this engine?
- b.) What total work did the engine do in 10,000 cycles?
12.During the expansion of a gas with constant mass and at constant pressure 9 MPa, the gas increased its volume from 0.2 m3 to 0.7 m3 and absorbed 6 MJ of heat in the process. Determine the change in the internal energy of the gas.
Please log in to view the solution.
13.Air with a given (unknown) mass and molar mass Mm = 29·10-3 kg·mol-1, which is in a cylinder with a movable piston, receives 5 kJ of heat in an isobaric process. What work does the air do in this process? How does the internal energy of the air change? (cp = 103 J·kg-1·K-1)
Please log in to view the solution.
14.The temperature of the cooler of a gas engine is 200C. The engine operates with an efficiency of 40%, which is 1.8 times less than the upper limit of efficiency. Determine the temperature of the heater.
Please log in to view the solution.
15.Steam is admitted into the cylinder of a steam engine at constant pressure of 3 MPa. The piston stroke is 0.3 m and the corresponding change in volume is 9·10-3 m3. What work does the steam do in one stroke? With what force due to pressure does the steam act on the piston?
Please log in to view the solution.
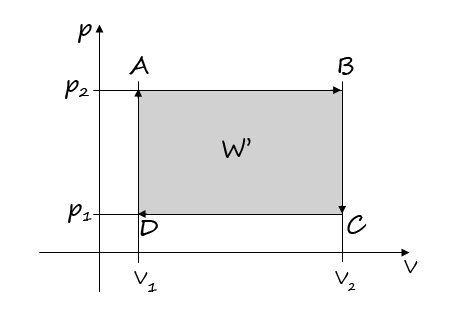
16.An ideal gas of constant mass undergoes the cyclic process ABCDA (diagram). What total work does the gas do in one cycle? Of which individual processes is this cycle composed? Solve for the values: