Molar mass
1. Calculate the mass in grams of one hydrogen molecule and of one hydrogen atom.
Solution:
The molar mass of molecular hydrogen (H2) is 2.016 g·mol-1. Substituting into the equation for calculating the mass per molecule gives:
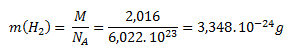
The molar mass of atomic hydrogen (H) is 1.008 g·mol-1. The mass of a single hydrogen atom is calculated analogously to the molecule:
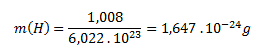
The mass of one hydrogen molecule is 3.348 × 10-24 g and of one hydrogen atom is 1.647 × 10-24 g.
2. For an experiment you need exactly 0.1 mol of zinc. What mass of zinc must be weighed?
Solution:
We start from the relation for molar mass. The molar mass of zinc from tables is 65.38 g·mol-1.
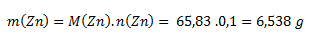
You need to weigh out 6.538 g of zinc.
3. Copper is a mixture of the isotopic nuclides 63Cu and 65Cu. The masses of the atoms of these nuclides are: 63Cu = 62.929 u and 65Cu = 64.928 u (u denotes atomic mass units—the numerical value of the relative atomic mass of an element and the mass of one atom of that element expressed in atomic mass units are the same). If the relative atomic mass of copper is 63.546, what is the percentage abundance of the two nuclides in the mixture?
Solution:
The given relative atomic mass of copper is the weighted average and its value depends on the percentage abundances of the two nuclides in the mixture. If we denote the percentage abundances as x and y, then the following equations apply:
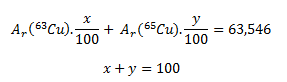
Because the numerical values of the atomic masses expressed in atomic mass units (u) are equal to the relative atomic masses, Ar(63Cu) = 62.929 and Ar(65Cu) = 64.928. Substituting these values into the equation gives:
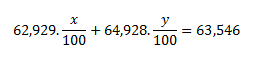
Substitute y = 100 − x
0.62929x + 0.64928(100 − x) = 63.546
Solving the equation gives the percentage abundance of nuclide 63Cu:
x = 69.13%
Abundance of nuclide 65Cu:
y = 100 − x = 100 − 69.13 = 30.87%
The mixture contains 69.13% of nuclide 63Cu and 30.87% of nuclide 65Cu.
4. Calculate the number of particles of the active substance in a homeopathic remedy prepared by the centesimal Hahnemann dilution C12. This is the procedure where from the mother tincture solution you take 1 part of solution and add 99 parts of pure solvent, mix, and repeat the step twelve times in total (dilution C12). The mother tincture consists of 1 g of NaCl dissolved in 1 liter of distilled water.
Solution:
We start from the equations for amount of substance and molar mass.
The mother tincture contains in 1 liter salt with mass m0 = 1 g; after the first dilution m1 = 0.01m0; after the second dilution m2 = 0.012m0; and after the i-th dilution mi = 0.01im0. The number of NaCl “molecules” Ni after the i-th dilution is:
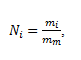 ,
,
where mm is the mass of a “molecule” of NaCl. The term molecule is not strictly correct here, since due to the ionic nature of the bond between Na and Cl the compound is present in solution as ions.
After substitution:
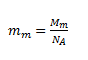
into the first equation we get:
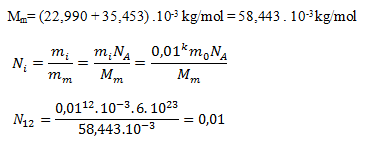
At the dilution level C12 there are 0.01 sodium cations per liter of water. In homeopathic treatment this is common—we are essentially taking a placebo without an active substance; any “effect” is psychological.
5. Determine the chemical equivalent of copper in an oxide in which copper is combined with oxygen in the mass ratio 3.971 : 1.000.
Solution:
The chemical equivalent of an element depends on the oxidation number of its atom in the compound. From the mass ratio of copper and oxygen we calculate the amounts of substance:
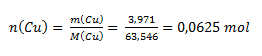
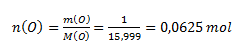
Since n(Cu) : n(O) = 1 : 1, the oxide has the composition CuO—copper(II) oxide. The Cu atom has oxidation number II and could thus bind two hydrogen atoms. One hydrogen atom corresponds to ½ atom of copper; therefore the chemical equivalent of copper in the oxide considered is ½ atom.
6. What must be the mass of calcium so that the sample contains the same number of atoms as there are in 10.00 g of zinc?
Solution:
Procedure as in example 2.
Result: 3.44 × 10-22 g
7. Natural lithium is a mixture of nuclides 6Li and 7Li with percentage abundances 7.54% and 92.46%. Calculate the relative atomic mass of nuclide 7Li, given that the relative atomic mass of nuclide 6Li is 6.016 and the average relative atomic mass of natural Li is 6.941.
Solution:
Procedure similar to example 3.
Result: 7.0142
8. The mass of one atom of a certain element is 3.158 × 10-22 g. Calculate the relative atomic mass (i.e., molar mass) of the element and determine which element it is.
Solution:
Multiply the atomic mass by Avogadro’s constant to obtain the mass of one mole of atoms, i.e., the molar mass of the element. The result is 190.21; from the periodic table we read that the element is Os (osmium).
9. Calculate the amount of substance of crystallization water in 50.4 g of iron(II) sulfate heptahydrate.
Solution:
The molar mass of water is 18 g/mol; for seven waters it is 7 × 18 = 126 g/mol. The molar mass of iron(II) sulfate heptahydrate is 277.909 g/mol.
In 277.909 g of iron(II) sulfate heptahydrate ................ 126 g of crystallization water
In 50.4 g of heptahydrate ................................................................. x g of water
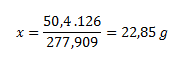
From that we calculate the amount of substance:
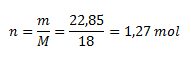
The amount of water in iron(II) sulfate heptahydrate is 1.27 mol.
10. Calculate the amount of substance:
- a) 5.32 × 1021 atoms of mercury (as in example 2)
- b) 50 g of mercury (as in example 2)
- c) of anhydrous calcium sulfate in 100 g of calcium sulfate dihydrate (as in example 9)
Solution:
- a) 8.83 mmol
- b) 0.249 mol
- c) 0.581 mol