Equations without the change of oxidation states
Non-redox equations are equations of chemical reactions in which the oxidation number of any atom does not change. There is no simple and universal algorithm to determine stoichiometric coefficients in non-redox reaction equations. Chemists initially approach solving non-redox equations mostly by trial and error, later improved by empirical methods, i.e., based on experience. However, the solution must always comply with the general rules for calculating stoichiometric coefficients. 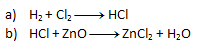
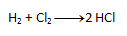
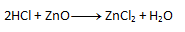
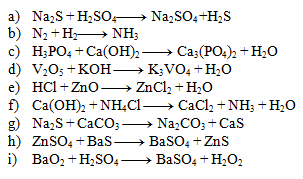
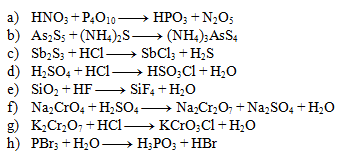
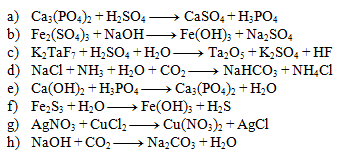
Please log in to view the solution.
Please log in to view the solution.
- At the beginning, we find in the chemical equation the substance (reactant or product) whose formula has the largest stoichiometric indices (i.e., contains the largest number of atoms). The stoichiometric coefficient of this substance will be considered as one.
- Then we add coefficients in front of substances that contain the same atoms as the substance with the assigned unit coefficient (according to the rule of atom count balance, or according to the rule of charge number balance).
- Gradually, we add coefficients in front of substances that do not yet have assigned coefficients based on the number of atoms in substances that already have coefficients (again according to the atom count balance rule or the charge number balance rule).
- Penultimately, we determine the number of hydrogen atoms and, if necessary, add stoichiometric coefficients.
- Finally, we determine the number of oxygen atoms on both sides of the equation. This operation usually serves to check the already obtained coefficients.
1. Balance the following chemical equations:
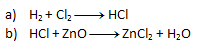
Solution:
- a) On the left side, both chlorine and hydrogen appear twice. According to the law of conservation of atoms, the same number must appear on the right side, so we write the number 2 in front of HCl. The resulting equation looks like this:
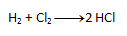
- b) On the right side, chlorine appears twice, but on the left side only once. Therefore, we write the number 2 in front of HCl. The number of other elements then "matches" on both sides.
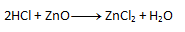
2. Balance the following chemical equations:
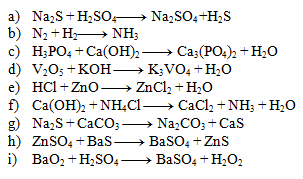
Solution:
- a) 1,1,1,1
- b) 1,3,2
- c) 2,3,1,6
- d) 1,6,2,3
- e) 2,1,1,1
- f) 1,2,1,2,2
- g) 1,1,1,1
- h) 1,1,1,1
- i) 1,1,1,1
3.
Fill in the stoichiometric coefficients for the following equations:
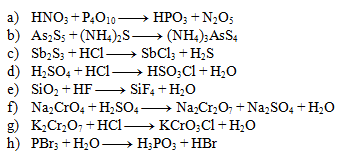
Solution:
- a) 4,1,4,2
- b) 1,3,2
- c) 1,6,2,3
- d) 1,1,1,1
- e) 1,4,1,2
- f) 2,1,1,1,1
- g) 1,2,2,1
- h) 1,3,1,3
4. Fill in the stoichiometric coefficients in the equations:
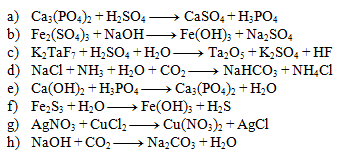
5. Determine the stoichiometric coefficients in the equation:
H3PO4 + CaCO3 → Ca3(PO4)2 + CO2 + H2O