isoprocesses
1. What are isoprocesses?
Solution:
Isoprocesses are processes in an ideal gas with constant mass, during which one of the state variables remains constant.
2.
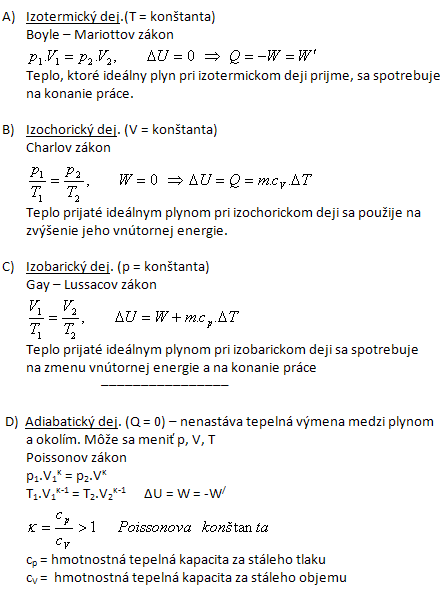
What volume will 30 liters of air have if, at the same temperature, we increase its pressure from 72 kPa to 75 kPa?
Solution:
Analysis:
V1 = 30 l = 30·10-3 m3, p1 = 72·103 Pa, p2 = 75·103 Pa, V2 = ?
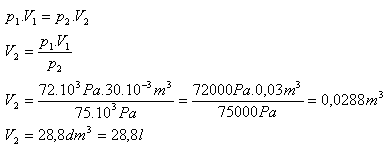
After increasing the pressure the air will have a volume of 28.8 liters.
3.
The density of helium at pressure 105 Pa is ρ1 = 0.164 kg·m-3. What will its density be if we compress it to a pressure of 5·107 Pa? (The mass and temperature of the gas do not change during compression).
Solution:
Analysis:
p1 = 105 Pa, ρ1 = 0.164 kg·m-3 , p2 = 5·107 Pa, ρ2 = ?
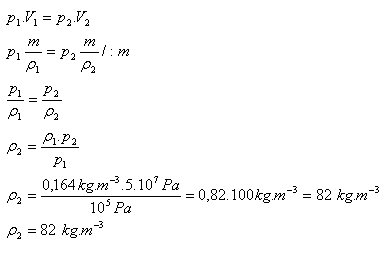
The density of helium after compression will be 82 kg·m-3.
4.
The density of air under normal conditions is ρ1 = 1.3 kg·m-3. What will the density of the air be if we heat it from normal conditions to 30oC? (The amount of air does not change).
Solution:
Analysis:
The density of the air will be 1.17 kg·m-3.
5.
A gas enclosed in a container with a movable piston is heated at constant pressure from 22oC to 52oC. By what percentage did its volume increase?
Solution:
Analysis:
T1 = 295.15 K, T2 = 322.15 K, Δ% = ?
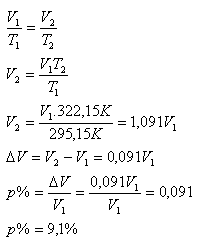
The volume of the gas after heating increased by 9.1%.
6. A bulb is filled with nitrogen during manufacturing at a pressure of 50.6 kPa and a temperature of 18oC. What is the temperature of the nitrogen in the lit bulb if its pressure increases to 118 kPa?
Solution:
Analysis:
p1 = 50.6 kPa = 50.6·103 Pa, T1 = 291.15 K, p2 = 118 kPa = 118·103 Pa, T2 =?
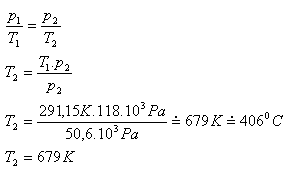
The temperature of the nitrogen in the lit bulb is approximately 679 K.
7.
At a Formula 1 race, the temperature of the air in the tires increased from 19oC to 79oC. How does the pressure in the tire change if it was originally inflated to 240 kPa? Why is a "formation lap" performed before the start? (The internal volume of the tire does not change)
Solution:
Analysis:
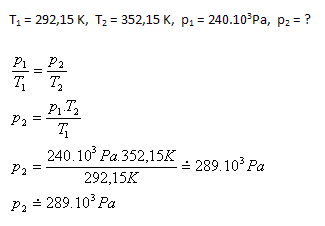
The air pressure in the tire after heating increases to approximately 289 kPa. The formation lap ensures that there is no sudden sharp increase in tire pressure and therefore in tire temperature right at the start of the race.
8.
In a cylindrical chamber there is air closed by a movable piston located 50 cm from the bottom of the cylinder. The air pressure is 105 Pa. If the piston is moved 20 cm toward the bottom during adiabatic compression, the air pressure increases to 2.05·105 Pa. Determine the Poisson constant for air!
Solution:
Analysis:
h1 = 0.5 m, V1 = 0.5S, h2 = 0.3 m, V2 = 0.3S, p1 = 105 Pa,
p2 = 2.05·105 Pa, κ = ?
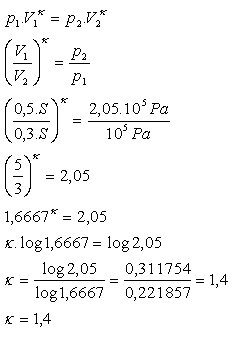
The Poisson constant for air is κ = 1.4.
9.
Show that for an adiabatic process T1·V1κ-1 = T2·V2κ-1
Solution:
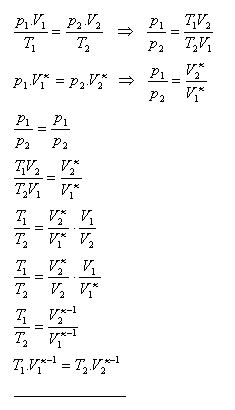
10. For adiabatic compression the volume of the air was reduced to 1/10 of the original volume. Calculate the pressure and temperature of the air after adiabatic compression. The initial air pressure was 105 Pa, the initial temperature 20oC. κ (air) = 1.4
Solution:
Analysis:
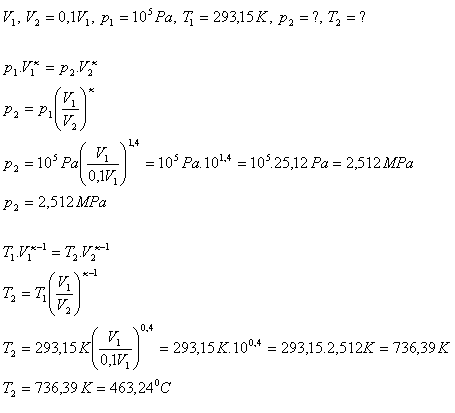
After adiabatic compression the air pressure increased to 2.512 MPa, the temperature to 463.24oC.