Compositions of solutions
1. How many grams of calcium nitrate and water are in 130 g of a 20% solution?
Solution:
100 grams of solution ........... 20 g of nitrate
130 g ......................................... x g of nitrate
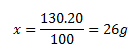
The mass of water is calculated as 130 − 26 = 104 g of water. The solution consists of 26 grams of calcium nitrate and 104 grams of water.
2. In 100 ml of water, 30 g of blue vitriol (copper(II) sulfate pentahydrate) were dissolved. What is the mass concentration of the solution thus prepared?
Solution:
First, it is necessary to calculate the amount of pure sulfate in blue vitriol.
M(CuSO4·5H2O) = 249.68 g·mol-1
M(CuSO4) = 159.60 g·mol-1
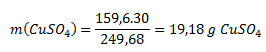
The mass of the solution is 100 g (water) + 30 g (blue vitriol) = 130 g. The concentration of the solution is therefore:
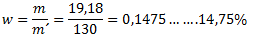
3. How many grams of sodium hydroxide are needed to prepare 5 liters of a 10% solution with density ρ = 1115 kg·m-3?
Solution:
We will use a combination of the formula for mass concentration and density:
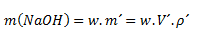
where m´ is the mass of the solution, V´ is the volume of the solution, and ρ´ is the density of the solution.
After substituting numerical values:
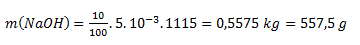
To prepare 5 liters of a 10% solution, we need 557.5 g of NaOH.
4. Calculate the molar concentration of a 22% sulfuric acid solution with density 1.1548 g·cm-3.
Solution:
From the density it follows that 1 liter of this solution weighs 1154.8 g. However, this solution contains only 22% pure acid, so its mass is calculated as follows:
m(pure acid) = 1154.8 · 0.22 = 254.848 g
The molar mass of sulfuric acid is 98 g·mol-1.
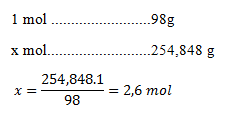
There are 2.6 mol of acid in one liter of solution, so the concentration is 2.6 mol·dm-3.
5. An aqueous ethanol solution contains 90 ml of pure alcohol in 150 ml of solution. What is the volume percent and mass percent of the solution?
Solution:
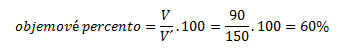
To calculate the mass percent, we need the densities of ethanol and of the solution — these are taken from chemical tables:
ρ100% = 0.7893 g·cm–3
ρ60% = 0.9094 g·cm–3
From these values we calculate the masses of such solutions:
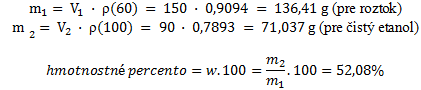
The composition of the solution is 60% (v/v) and 52.08% (w/v), respectively.
6. How many grams of potassium chloride and how many milliliters of water are needed to prepare 245 g of a 2.5% solution?
Solution:
We will use the rearranged relation for calculating mass concentration:
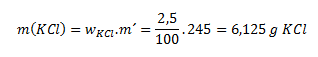
The amount of water is calculated as 245 g (solution) − 6.125 g (chloride) = 238.875 g of water.
To prepare the solution we need 6.125 grams of potassium chloride and 238.875 g of water, which according to the unit density of water corresponds to 238.875 ml of water.
7. What is the mass of potassium permanganate present in 25 ml of a 0.02 molar solution?
M(KMnO4) = 158.342 g·mol-1.
Solution:
We use the rearranged relation for amount concentration:
m = c · M · V = 0.02 · 158.342 · 0.025 = 0.0792 g
The mass of permanganate is 0.0792 g.
8.
What amount concentration will a solution of ammonium iron(III) sulfate have if 1 ml of such a solution corresponds to 1 mg of Fe2O3? M(Fe2O3) = 159.7 g·mol-1.
Solution:
If 1 ml of NH4Fe(SO4)2 solution corresponds to 1 mg of Fe2O3, then 1000 ml of this solution corresponds to 1 g of Fe2O3. The amount concentration of the oxide is:
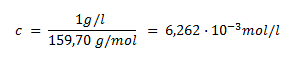
Because 1 mol Fe2O3 ≈ 2 mol NH4Fe(SO4)2, the ammonium iron(III) sulfate solution will be 0.012524 molar
(twice as concentrated as the Fe2O3 solution).
9. What is the mass fraction and percent composition of a solution that contains, in 10 ml of water, 1.02 of dissolved substance?
Solution:
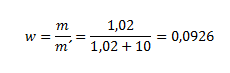
mass percent = w · 100 = 0.0926 · 100 = 9.26%
The mass fraction of the dissolved substance is 0.0926; the mass percent is 9.26%.
10. What are the amount concentration and mass concentration of a nitric acid solution with a mass percent of 40? ρ40 = 1.252 g·cm-3, Mr(HNO3) = 63.01.
Solution:
The mass of HNO3 in 1 liter is calculated as:
m = 0.40 · 1252 = 500.8 g
Mass concentration:
δ = 500.8 / 1 = 500.8 g·L-1
Amount concentration:
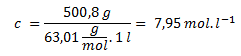
The mass concentration is 500.8 g·L-1, and the amount concentration is 7.95 mol·L-1.
```