Stochiometry
Stoichiometry deals with the quantitative relationships between atoms of elements in compound molecules and the relationships between substances that participate in chemical reactions.
Empirical formula indicates, using small whole numbers, the ratio in which the atoms of elements are present in the compound they form.
Molecular formula indicates the number of atoms in a molecule, e.g. the molecular formula of hydrogen peroxide is H2O2 and the empirical formula is HO. The molecular formula is either identical to the empirical one or is its integer multiple.
The most common ways of expressing the composition of compounds are:
- Mass ratio of the elements forming the compound. For a compound of composition AxByOz the following holds:
m(A) ∶m(B) ∶m(O)=M(A).x ∶ M(B).y ∶ M(O).z, where m are the masses and M are the molar masses of the elements A, B, and O.
- Mass fraction wB indicating what mass of component B corresponds to a unit mass of the compound.
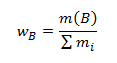 ,
,
where mi is the mass of the individual components of the compound. The sum of the mass fractions of all components that make up the compound equals 1:
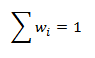
- Mass percent, which expresses the mass of component B per 100 g (kg) of the compound.
mass percent= 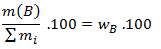
- Mole fraction xB expressing what amount of substance of component B corresponds to the total amount of substance of all components. The mole fraction is defined by the relation:
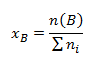
where ni are the amounts of substance of the individual components of the compound. The sum of the mole fractions of all components of the compound equals 1:
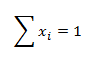
- Volume fraction of the i-th component φi is the ratio of the volume of the i-th component Vi to the volume of the whole system V.
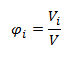
- Volume percent (vol.%) is obtained by multiplying the volume fraction by 100:
vol.%=φi.100