States of matter
1.
Explain the states of matter and their changes!
Solution:
Substances can exist in three states (phases) – solid, liquid, and gas.
Melting – change from solid to liquid
Freezing – change from liquid to solid
Evaporation – change from liquid to gas
Boiling – evaporation occurring when the vapor pressure equals the external pressure
Condensation – change from gas to liquid
Sublimation – direct change from solid to gas
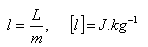
L = latent heat (melting, freezing, evaporation, boiling ...)
l = specific latent heat (melting, freezing, evaporation, boiling ...)
m = mass of substance
For ice – water – steam applies:
t melt = t freeze = 00C, t boil = t condense = 1000C,
l melt = l freeze = 334.103J.kg-1, l boil = l condense = 2260.103J.kg-1
l evap(200C) = 2430.103J.kg-1, l sub = 2830.103J.kg-1
c(H2O) = 4180 J.kg-1.K-1, c(ice) = 2100 J.kg-1.K-1
Conversion of ice to steam: Q = Q1 + L1 +Q2 + L2 + Q3
Q1 = heat needed to warm ice to melting point
L1 = heat needed to convert ice to water
Q2 = heat needed to warm water to boiling point
L2 = heat needed to convert water to steam
Q3 = heat needed to warm steam
2.
Calculate the heat required to melt an aluminum object with a mass of 10 kg and an initial temperature of 200C. Use the tables.
Solution:
Analysis:
m = 10 kg, t1 = 200C, tm (Al) = 6600C, c(Al) = 896 J.kg-1.K-1
lm (Al) = 400.103J.kg-1
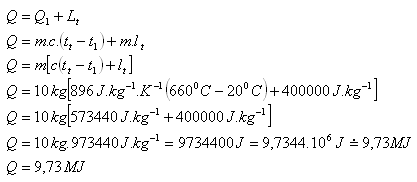
To melt the aluminum object, approximately 9.73 MJ of heat is required.
3.
Determine the specific latent heat of fusion of copper if it is known that melting 5 kg of copper heated to its melting point requires 1.02 MJ of heat.
Solution:
Analysis:
m = 5 kg, L = 1.02.106J,
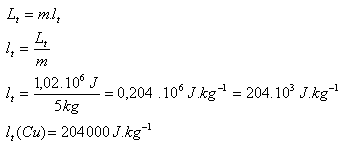
The specific latent heat of fusion of copper is lm (Cu) = 204,000 J.kg-1
4.
Ice is placed into 5 liters of water at 500C. What must be the mass of this ice so that it all melts and the final water temperature after melting is 00C? Use the tables!
Solution:
Analysis:
m1 = 5 l = 5 kg, t1 = 500C, Δ t = 500C, c1(H2O) = 4180 J.kg-1.K-1
lm (ice) = 334,000 J.kg-1
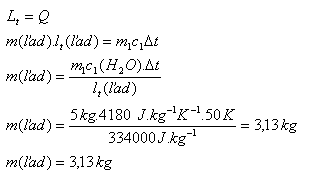
The mass of ice must be m = 3.13 kg.
5.
Water with a mass of 10 kg and a temperature of 00C is heated to 1000C and then completely evaporated into steam at the same temperature. What is the total heat absorbed by the water? What percentage of this heat is used to heat the water and what percentage for the phase change? Use the tables!
Solution:
Analysis:
m = 10 kg, Δt = 1000C = 100 K, c1 = 4180 J.kg-1.K-1,
lv = 2.26.106 J.kg-1