Solutions
Solutions are homogeneous mixtures of two or more substances. They have variable composition depending on the mutual solubility of the components in the system. Every solution contains a solvent and a dissolved substance. Usually, the higher the temperature, the greater the amount of substance that can be dissolved in the solvent (solubility of the substance increases with temperature).
The composition of solutions can be expressed in the following ways:
Mass fraction wB indicates what mass of component B corresponds to the unit mass of the solution:
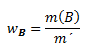
where m(B) is the mass of component B, and m´ is the mass of the solution.
Mass percent expresses what mass of component B is contained in 100 g (kg) of the solution. It is determined by the ratio of the mass of component B to the mass of the solution, multiplied by one hundred:
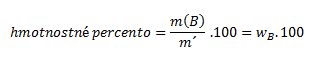
Volume fraction φB indicates the volume of substance B corresponding to the unit volume of the solution:
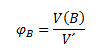
Volume percent expresses what volume of component B is contained in 100 ml (l) of the solution:
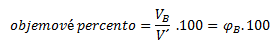
Mole fraction xB expresses what amount of substance (in moles) of component B corresponds to the unit total amount of substance of all components in the solution:
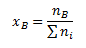
Substance concentration (amount concentration, molarity) cB, which is simply denoted as M.
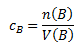
Molality mB expresses the amount of substance B dissolved in 1 kg of solvent:
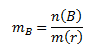
where m(r) is the mass of the solvent expressed in kilograms; the unit of molality is mol·kg-1.
When considering mass balances in solutions, we take into account the following situations:
- a.) Dilution of a solution by adding pure solvent: This increases the total mass of the solution, but the total mass of the dissolved substance remains constant. When calculating the composition of the resulting solution or the amount of the initial solution required, we use the so-called mass balance method. We draw a diagram of the preparation of the resulting solution; water with zero concentration of the dissolved substance and a solution with a known concentration of the dissolved substance are added to the system:
Balance diagram:
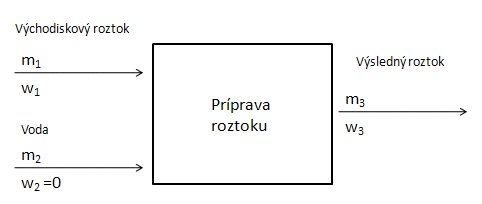
We use the following equation:

- b.) Preparation of a solution by mixing two solutions of different concentrations: The resulting mass of the solution m´₃ is the sum of the masses of the mixed solutions. The amount of dissolved substance is also given by the sum of the masses of the substances in the initial solutions:
Balance diagram:
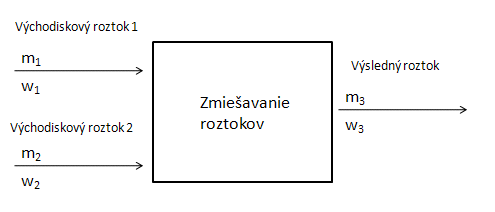
We use the following equation:
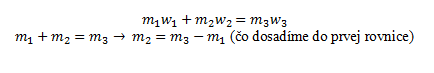
- c.) Preparation of a solution by evaporation of the solvent: This reduces the total mass of the solution, but the amount of dissolved substance remains the same:
Balance diagram:
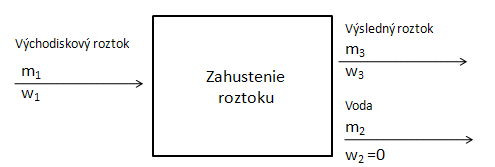
We use the following equation:
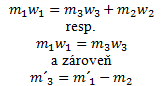
- d.) Preparation of a solution by crystallization: Cooling the solution decreases solubility, and the excess substance separates from the solution in the form of crystals:
Balance diagram:
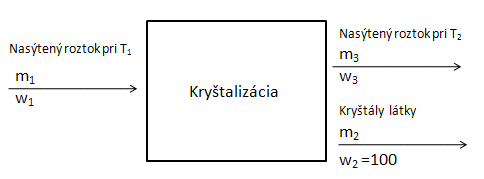
We use the following equation:
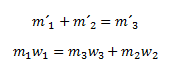
```