Electric current in the electrolytes
1.Describe the electric current in electrolytes!
Solution:
Electrolytes are substances whose solutions or melts conduct electric current by means of ions. Electric current in electrolytes is an ordered movement of cations (+) and anions (-). Cations move toward the cathode (-), anions toward the anode (+). Electrolytic dissociation is the process in which neutral molecules of an electrolyte split into ions. Electrolysis is the process during which chemical changes occur at the electrodes and in the electrolyte when electric current passes through it.
1. Faraday’s law:
electrochemical equivalent
2. Faraday’s law:
Faraday constant
Mm = molar mass
oxidation number (from tables)
3. Combined Faraday’s law:
Ohm’s law in electrolyte:
decomposition voltage
2.Calculate:
- a.) the amount of aluminum that is deposited at the electrode during electrolysis in 24 hours by a current of 10 kA. A(Al3+) = 0.093·10-6 kg·C-1
- b.) what current would deposit chromium with a mass of 3.24 g from the electrolyte in 1 hour. A(Cr3+) = 0.18·10-6 kg·C-1
Solution:

- a.) 80 kg of aluminum is deposited at the electrode.
- b.) Chromium is deposited at the electrode by a current of 5 A.
3.Determine the electrochemical equivalent of aluminum and copper
Solution:
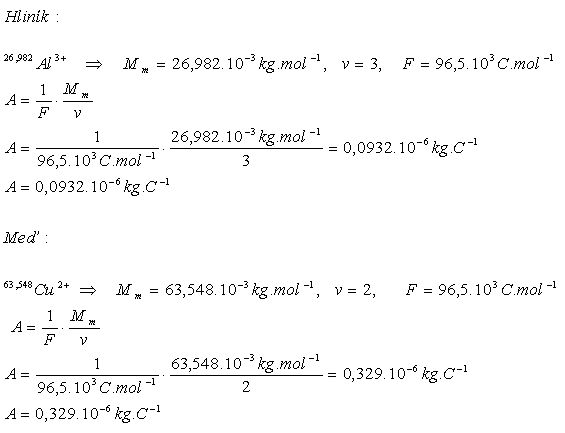
The electrochemical equivalents are A(Al)= 0.0932·10-6 kg·C-1 and A(Cu)=0.329·10-6 kg·C-1
4.An electric current of 1 A passes through an electrolyte of CuSO4. How many copper atoms are deposited on the cathode in 10 seconds? A(Cu) = 0.329·10-6 kg·C-1, NA = 6.022·1023 mol-1, Mm = 63.548·10-6 kg·mol-1
Please log in to view the solution.5.A sphere with radius r = 5 cm is to be nickel-plated with a layer thickness h = 0.15 mm. How long should the sphere remain in the electrolyte at a current I = 1 A?
Please log in to view the solution.
6.Nickel-plating of a metal plate with surface area 100 cm2 took four hours at a current of 0.4 A. Calculate the thickness of the nickel layer formed on the plate!
Please log in to view the solution.7.How much energy do we need to obtain 1 g of copper by electrolysis of copper sulfate CuSO4, if the electrolysis runs at a voltage of 4 V?
Please log in to view the solution.
8.Two electrolytic cells with solutions of AgNO3 and CuSO4 are connected in series. How much copper is deposited in the time during which 850 mg of silver is deposited?
Please log in to view the solution.9.What electric current passed through the electrolyte if the copper cathode weighed 35 g before the measurement and 39 g after the measurement, which lasted 40 minutes?
Please log in to view the solution.
10.The decomposition voltage during electrolysis of sulfuric acid was 2.2 V. At a voltage of 3.5 V, a current of 2.8 A passed through the electrolyte. The electrodes were 4 cm apart and the cross-sectional area of the electrolytic conductor was 30 cm2. Determine the resistivity of the electrolyte!
Please log in to view the solution.
11.In what time will copper with a mass of 1.778 g be deposited from a CuSO4 solution by a current of 3 A?
Please log in to view the solution.12.During the electrolysis of blue vitriol CuSO4, divalent copper with a mass of m = 9.504 g is deposited on the cathode in 2 hours. The ammeter showed a current IA = 4.1 A. Determine the error of the ammeter ΔI.
Please log in to view the solution.13.Two electrolytic vessels with AgNO3 and CuSO4 solutions are connected in series. Determine the mass of copper that will be deposited during the time in which 180 g of silver is deposited in the other vessel.
Please log in to view the solution.14.During the electrolysis of ZnSO4, zinc with mass m = 2.45 g is deposited in 1 hour. Determine the electrical resistance of the solution if the voltage on the electrodes was U = 6 V.
Please log in to view the solution.15.An object with a surface area S = 20 dm2 needs to be silver-plated with a layer thickness h = 0.2 mm. How long will the plating take if 1 dm2 of surface can be loaded with a current of I = 0.4 A?
Please log in to view the solution.
16.How much energy is needed to obtain copper with a mass m = 1 g during the electrolysis of CuSO4, if the electrolysis was performed at a voltage U = 4 V?
Please log in to view the solution.17.A current I = 1 A passes through a CuSO4 solution. How many copper atoms are deposited on the cathode in t = 1 s? (z = 2)
Please log in to view the solution.18.What is the electric charge of one silver ion that is deposited during the electrolysis of silver nitrate by a charge Q = 1 C?
Please log in to view the solution.19.How much a.) silver b.) divalent copper will be deposited from their respective solutions if a charge Q = 96.5·106 C passes through them?
Please log in to view the solution.
20.The accumulator was charged with a current I1 = 5 A for a time t1 = 6 hours. How long did it discharge if a steady current I2 = 0.05 A was drawn during discharging? The charge efficiency of the accumulator is η = 90%.
Please log in to view the solution.