Internal energy
1. What is the internal energy of a system and how can it be changed?
Solution:
A thermodynamic system is a collection of microscopic and macroscopic objects forming a whole that can exchange energy with each other or with the surroundings. The total energy of the system is the sum of the total kinetic energy of the disordered motion of particles and the total potential energy of the mutual positions of those particles.
U = Ek + EP
First law of thermodynamics:
The change of the internal energy of the system ΔU equals the sum of the work W done on the system by surrounding bodies acting with forces and the heat Q delivered to the system by the surroundings.
ΔU = W + Q
- a.) The system receives energy (work, heat): W > 0, Q > 0
- b.) The system gives off energy (work, heat): W < 0, Q < 0
Change of internal energy caused by external work: (Q = 0)
Change of internal energy by heat exchange: (W = 0)
Heat capacity:
Specific heat capacity (tables):
Amount of heat received (or given) by a system:
Calorimetric equation (mixing rule):
With calorimeter:
2.English physicist J. P. Joule (1818 – 1889) attempted to determine the specific heat capacity of water. In his experiment he used two weights, each of mass 14 kg, and let them fall 12 times in a row from a height of 2 m into a container with water. The container held water of mass 6.7 kg. During the experiment the temperature of the water increased by 0.24 K. What value of the specific heat capacity of water did he find?
Solution:
Analysis:
2.m1 = 28 kg, Ep =2.m1.g.h = 28kg.10m.s-2.2m = 560 J,
Ek = 12.Ep = 12.560J = 6720 J, m2 = 6,7kg, Δt = 0,24 K
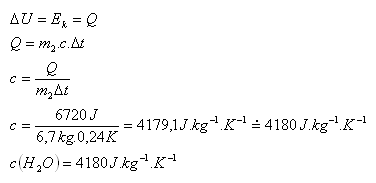
Specific heat capacity of water c(H2O) = 4180 J·kg-1·K-1
3.Think about a short test: If T(K) = t(°C) + 273.15, then which of the statements is true?
Temperature 0oC is
A) –100oK B) 0oK C) 273.15 K
2) Temperature -273.15 is
A) the freezing temperature of helium B) absolute zero C) nonsense
3) Temperature 185.2 oC is
A) –85.2 K B) 458.35 K C) 358.35 K
4) Temperature 0 K is
A) nonsense B) –273.15oC C) 273.150oC
5) A pupil said “The temperature of the water increased by 20oK.” In reality it increased by
A) 20oC B) 293.15oC C) 253.15oC
6) Temperature –1273.15oC is
A) the freezing temperature of hydrogen B) nonsense C) 73.15 K
7) Temperature 290 K is
A) the temperature of a healthy person B) 290oC C) 16.85oC
8) Temperature 373.15 K is
A) boiling temperature of water B) nonsense C ) 0oC
Solution:
1C, 2B, 3B, 4B, 5A, 6B, 7C, 8A
4.A steel rod has heat capacity C = 1.5·103 J·K-1. How will its temperature change if
- a) it absorbs heat Q1= 25·103 J
- b) it gives off heat Q2 = 0.45·106 J
5.Calculate how long it takes for water to heat from 15oC to the boiling temperature when heated by an immersion heater, if the volume of water is 150 cm3. The heater power is 500 W and its efficiency is 95%. c(H2O) = 4180 J·kg-1·K-1.
Please log in to view the solution.6.To water with mass 2.5 kg and temperature 15oC a steel cylinder of mass 0.9 kg at temperature 300oC was inserted. What will be the final temperature of the water and the cylinder after reaching equilibrium?
Please log in to view the solution.
7.Five steel plates with a total mass of 7 kg were heated to 910oC and immersed into oil at 10oC. How many liters of oil must we use in the quenching bath so that the bath temperature stabilizes at 190oC.
Please log in to view the solution.
8.There is 0.42 kg of water at 20oC in a container. If we pour another 0.9 kg of water at 70oC into the container, we find that the final temperature after reaching equilibrium is 50oC. What is the heat capacity of the container?
Please log in to view the solution.
9.Into a container with C = 2 J·K-1 was poured water of mass 0.4 kg and temperature 17oC. A cylinder of unknown material with mass 0.124 kg and temperature 64oC was placed into the container. The system temperature equilibrated at 19oC. What material is the cylinder made of? Use tables!
Please log in to view the solution.
10.When mixing 20 liters of water at 12oC with 40 liters of water at 80oC, 420 kJ of heat escaped into the air. Determine the final temperature of the water!
Please log in to view the solution.
11.A body with a mass of 1 kg slides down an inclined plane 21 m long with a slope of 300. The speed at which the body leaves the inclined plane is 4.1 m·s-1. Determine the total change in internal energy of the body and the inclined plane.
Please log in to view the solution.
12.Water with a mass of 1 kg was heated from a temperature of 00C to 1000C and absorbed a certain amount of heat. Compare this heat with:
b.) the kinetic energy of this body at a speed of 100 m·s-1.
13.How much heat is absorbed by oil (c = 1.7·103 J·kg-1·K-1) with a volume of 2 l and density 910 kg·m-3 when heated from 200C to 650C?
Please log in to view the solution.
14.During turning (machining), both the cutting tool and the workpiece heat up, which is why they must be cooled by a coolant. The thermal power of the coolant is 50 kJ·min-1. How many liters of coolant are needed for one hour of turning if its density is 980 kg·m-3, initial temperature 200C, final temperature 600C and c = 3.9 kJ·kg-1·K-1?
Please log in to view the solution.
15.A lead bullet flying at a speed of 100 m·s-1 hit a stationary wooden board and lodged in it. Determine the temperature increase of the bullet if 50% of its kinetic energy is converted into its internal energy upon impact. (c(Pb) = 129 J·kg-1·K-1)
Please log in to view the solution.
16.An automobile has a four-cylinder engine with power P = 52 kW. Determine the efficiency of the engine if at a speed v = 120 km·h-1 it consumes 15 liters of gasoline over a distance of 100 km.
Please log in to view the solution.
17.Determine the mass of gunpowder needed for a bullet with a mass of 50 g to reach a height of 2 km when fired vertically upward. Efficiency is 15%, calorific value 2.94 MJ·kg-1. (Air resistance neglected)
Please log in to view the solution.
18.In a vertical cylinder, a gas is enclosed by a movable piston with a mass of 1.5 kg. The gas absorbed 13 J of heat from the surroundings and simultaneously lifted the piston by 20 cm. How did its internal energy change?
Please log in to view the solution.
19.In an iron container (cFe = 452 J·kg-1·K-1) with a mass of 0.1 kg there is water (cH2O = 4180 J·kg-1·K-1) with a mass of 0.5 kg and temperature 150C. An aluminum (cAl = 896 J·kg-1·K-1) and a lead (cPb = 129 J·kg-1·K-1) body with a total mass of 0.15 kg and temperature 1000C were placed into the container. After reaching equilibrium, the water temperature increased to 170C. Determine the mass of the aluminum body (x) and the mass of the lead body (y).
Please log in to view the solution.